
Understanding the Properties and Applications of Calcium Hydroxide and Its CAS Number Identification
Understanding Calcium Hydroxide CAS Number and Its Applications
Calcium hydroxide, commonly referred to as hydrated lime or slaked lime, is an inorganic compound with the chemical formula Ca(OH)₂. Identified by its CAS number 1305-62-0, this compound is a white powder that is sparingly soluble in water, producing an alkaline solution known as limewater. Calcium hydroxide plays a significant role in various industrial and environmental applications, making it an essential chemical in many fields.
One of the primary uses of calcium hydroxide is in the construction industry. It is a key component in the production of lime mortar, plaster, and cement. When mixed with water, calcium hydroxide reacts with carbon dioxide in the atmosphere to form calcium carbonate, a process that contributes to the durability and strength of building materials. This reaction enhances the properties of mortars and plasters, making them more resistant to weathering and wear over time.
Understanding Calcium Hydroxide CAS Number and Its Applications
In agriculture, calcium hydroxide serves as a soil amendment that helps improve soil quality and promote healthier crop yields. By raising the soil pH, it mitigates acidity, fostering a more favorable environment for plant growth. Additionally, using calcium hydroxide can enhance nutrient availability in the soil, enabling crops to absorb essential minerals effectively. This property makes it a vital component in sustainable agricultural practices, aiding farmers in maximizing their productivity.
calcium hydroxide cas number
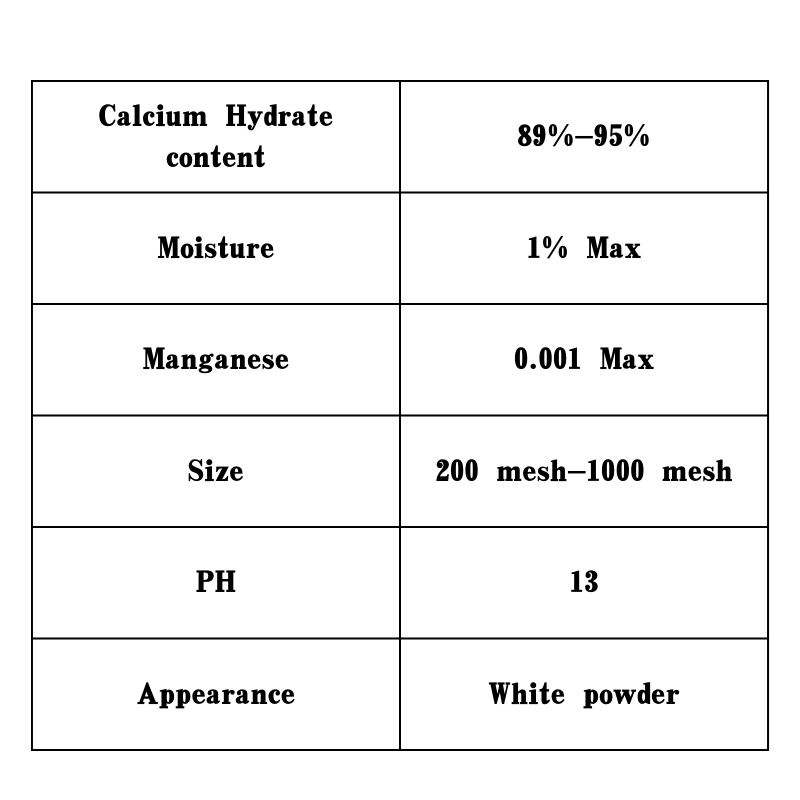
Beyond its industrial and agricultural applications, calcium hydroxide is also utilized in the food industry. It can act as a food additive, serving as a pH regulator and firming agent in various food products. For instance, in the production of tortillas and corn products, calcium hydroxide is used in a process called nixtamalization. This method improves the nutritional value of corn by making niacin more bioavailable and enhancing the flavor and texture of the final product.
In the field of medicine, calcium hydroxide is employed in dental applications, particularly in endodontics. It is known for its antibacterial properties and is utilized as a pulp capping agent to promote healing in exposed dental pulp tissues. Furthermore, its ability to stimulate reparative dentin formation makes it an effective material for maintaining tooth vitality in various dental treatments.
Despite its numerous benefits, it's essential to handle calcium hydroxide with care. The compound can be caustic and may cause irritation to the skin, eyes, and respiratory tract if proper safety precautions are not followed. Therefore, users should wear appropriate protective equipment and adhere to safety guidelines during its handling and application.
In summary, calcium hydroxide (CAS number 1305-62-0) is a versatile compound with extensive applications in construction, water treatment, agriculture, food processing, and medicine. Its unique properties enable it to serve as a critical component in improving material durability, enhancing soil quality, and contributing to health and safety practices. As industries continue to evolve, the significance of calcium hydroxide remains paramount, underscoring its integral role in a wide array of sectors.
Share
-
Premium Talcum Powder Enhanced with GPT-4 Turbo | Soft & Long-LastingNewsAug.02,2025
-
Fly Ash Solutions Enhanced by GPT-4 Turbo | Sustainable InnovationNewsAug.01,2025
-
Natural Premium Bentonite Cat Litter - Superior ClumpingNewsJul.31,2025
-
Premium Resin Coated Sand - High Heat Resistance CastingNewsJul.31,2025
-
High Quality Silicon Carbide Grit for Abrasive ApplicationsNewsJul.30,2025
-
High-Quality Ceramsite for Plants & Gardening | Lightweight PebblesNewsJul.29,2025






